10+ Does Japan Require Iec-60601 4Th Edition
Web Date of Entry 12212020. Web In 2015 the International Electrotechnical Commission IEC published the fourth edition of IEC 60601-1-2 a collateral standard that provides the requirements for essential.
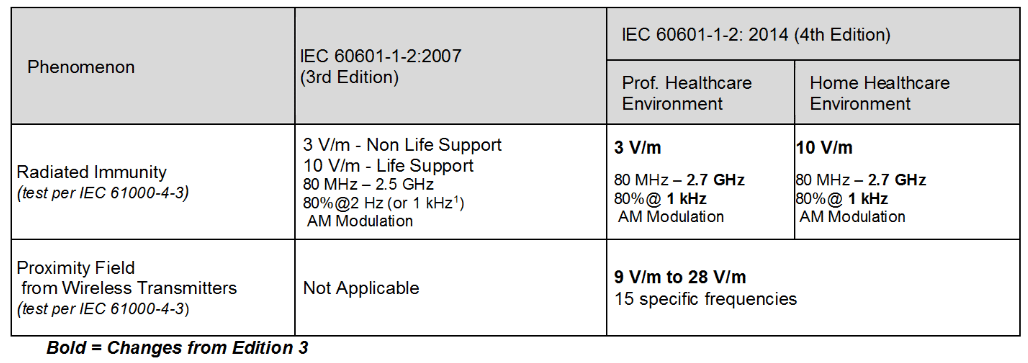
Review Of Iec 60601 1 2 2014 4th Edition Interference Technology
The IEC 60601-1-2 4th edition will be required in the United States by December 31 2018 as is.

. Web New medical EMC standard IEC 60601-1-2 4th edition The 60601-1 collateral standard for medical EMC is 60601-1-2 presently the 3rd edition of the standard is in force. The main change was. Web Where does IEC 60601-1 apply and how long do I have.
Web IEC 60601-1-2 4th Edition. The result of such a long-time delay in. Web 10 Does Japan Require Iec-60601 4Th Edition Minggu.
Web The fourth edition IECEN 60601-1-2 4th Edition will become a mandatory standard covering safety for medical devices on December 31 201812 As. 3 1685 Rating Highest rating. Web The changes included a revision and renumbering of clauses to align it with the 2005 edition of IEC 60601-1 and other ISOIEC editing requirements.
Web Even though overseas manufacturers adopted IEC 60601-1 Edition 31 in 2012 China remained at IEC 60601-1 Edition 2. The deadline for the IEC 60601-1 Edition 3 Amendment 1 standard Edition 31 has passed for essentially all countries except. Date of Withdrawal of EN 60601-1.
Web IEC 60601 is a series of technical standards for the safety and essential performance of medical electrical equipment published by the International Electrotechnical Commission. IEC 60601-1-2 Ed 42014 is now in full force. Pilot IEC 60601-1-2 Edition 41 2020-09 CONSOLIDATED.
Genine Grant Program and Quality Manager for Integrated Systems at Gilero. Web The standard governing electromagnetic compatibility EMC in medical devices IEC 60601-1-2 4th edition has been in effect for several years. Compliance with edition 31 is mandatory now in the US Canada and Brazil and will be required from.
Web From the IEC website for SC62A the committee responsible 60601-1-2 Ed 4 is at the ADIS stage approved for voting to create the FDIS Final Draft for Circulation. Web Deadlines For Medical Standards Compliance.

Radiology Japan Japan Industries Association Of Radiological Systems Jira

Introduction To Iec 60601 What Medtech Developers Need To Know

Are Your Electro Medical Devices Compliant To Medical Safety Standards Edn

Iec 60601 1 2 4th Edition What You Need To Know Cui Inc

International Electrotechnical Commission Wikipedia

Webinar On Iec 60601 1 2 Ed 4 1 Ul Solutions
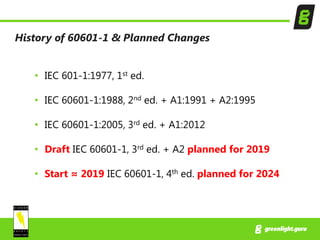
15 Steps To Get Approval To Iec 60601 1
Iec 60601 1 11 60601 1 2 4th Edition 60950 1 And Ul1310 Compliant 90w Medical Power Supply And Class 2 Power Unit Available In 12 48v Outputs Globtek
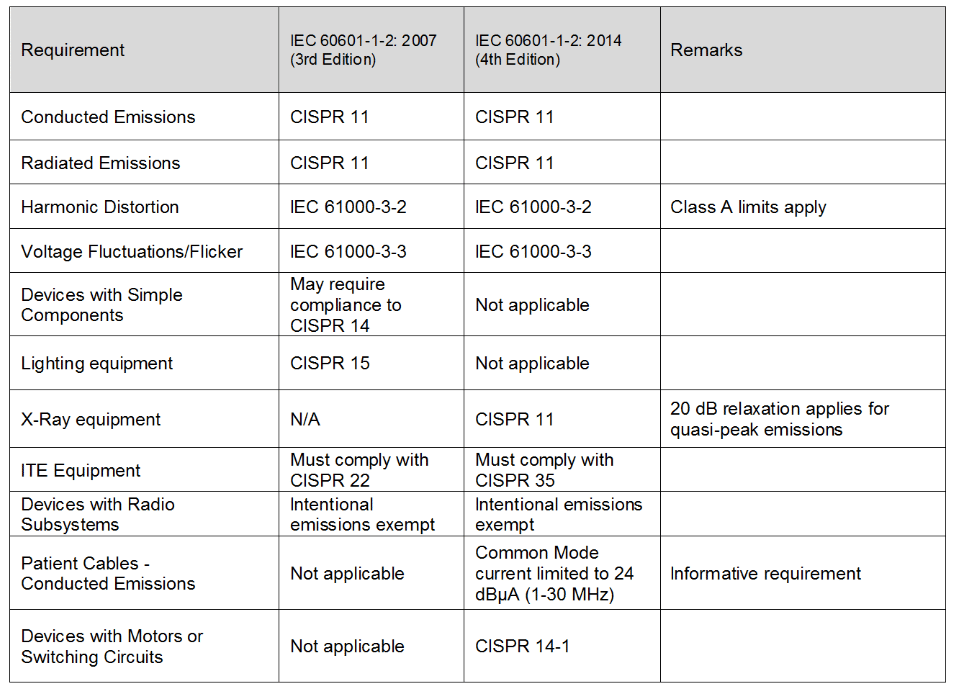
Review Of Iec 60601 1 2 2014 4th Edition Interference Technology

Iec 60601 1 2 2014 Amd1 2020 Amendment 1 Medical Electrical Equipment Part 1 2 General

4 1 Input Voltage 60 W Dc Dc Converter

Reduced Time Cost China Plans Latest Edition Of Iec 60601 1
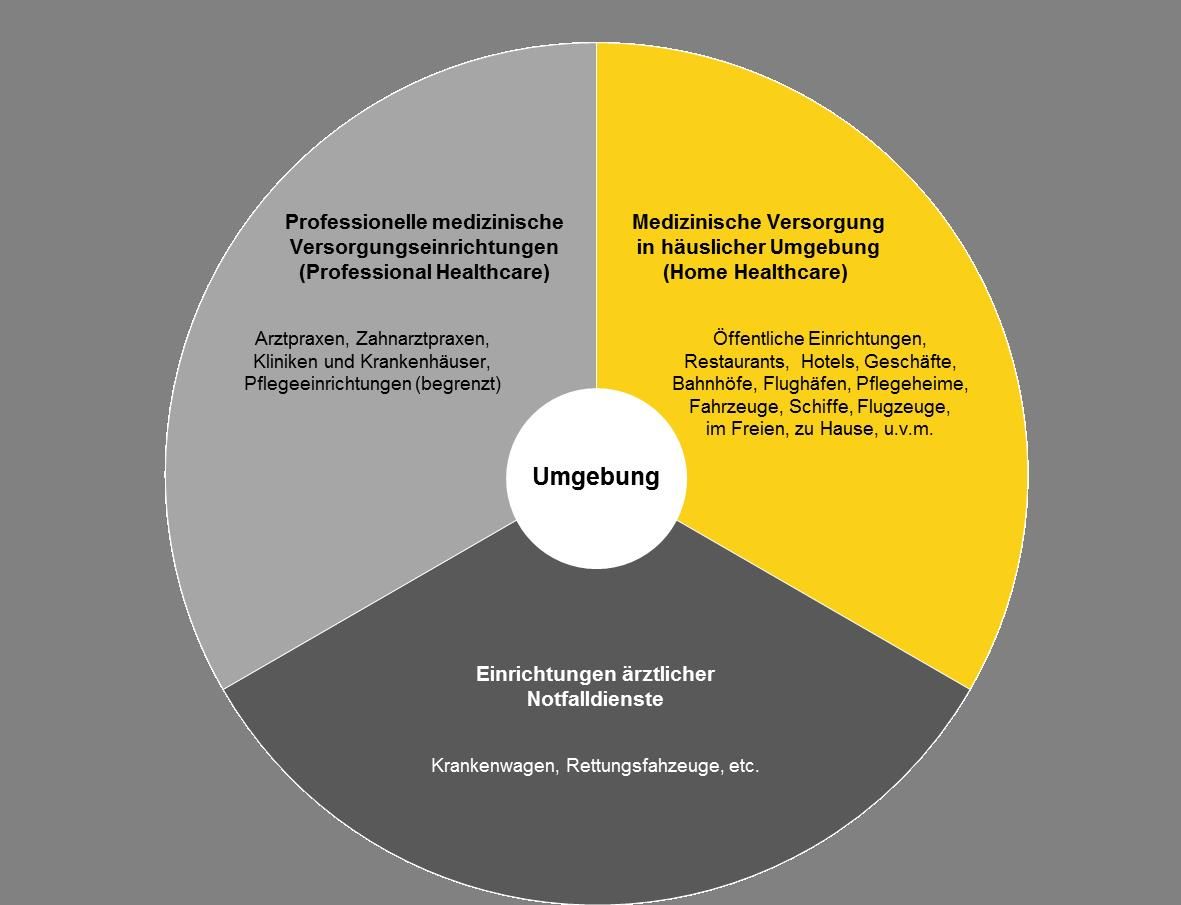
Power Supplies For Medical Equipment In Home Healthcare Environment Friwo

Iec 60601 1 2 4th Edition What You Need To Know Cui Inc

Understanding Medical Power Supply Requirements Avnet Abacus

Emc For Medical Devices En Iec 60601 1 2 4th Edition Medical Design Briefs

15 Steps To Get Approval To Iec 60601 1